The only FDA-approved enzyme replacement therapy indicated for the treatment of sucrase deficiency, which is part of congenital sucrase-isomaltase deficiency (CSID), in adult and pediatric patients 5 months of age and older.1

How Sucraid® Works
Sucraid®: The mechanism of action.
The active ingredient in Sucraid® is sacrosidase, which is a sucrase enzyme replacement. It splits sucrose into glucose and fructose, which facilitates absorption of both into the bloodstream from the small intestine.1 Although Sucraid® provides replacement therapy for the deficient sucrase, it does not provide specific replacement therapy for the deficient isomaltase.
Dosage Information
Recommended dosage of Sucraid®.
Each dose should be diluted in 4 ounces of water, milk, or infant formula, and can be measured with the provided 1-mL measuring scoop.1
1 mL (8,500 IU; one full measuring scoop) per meal or snack for infants, toddlers, and small children weighing up to 15 kg (33 lb)1
2 mL (17,000 IU; two full measuring scoops) per meal or snack for older children and adults weighing over 15 kg (33 lb)1
- Half of each Sucraid® dose should be taken before a meal and the other half during a meal.1
- The beverage or infant formula used to dilute Sucraid® should not be heated, nor should Sucraid® be added to hot beverages, as heat can decrease the potency of the enzyme.1
- Sucraid® should not be reconstituted or consumed with fruit juices as the acidity may impair enzyme activity.1
- Sucraid® should be refrigerated at 36°F to 46°F (2°C to 8°C) and protected from heat and light.1
- Bottles of Sucraid® should be discarded four weeks after first opening due to the potential for bacterial growth.1
Discover the Convenience of the Sucraid® Single-Use Container
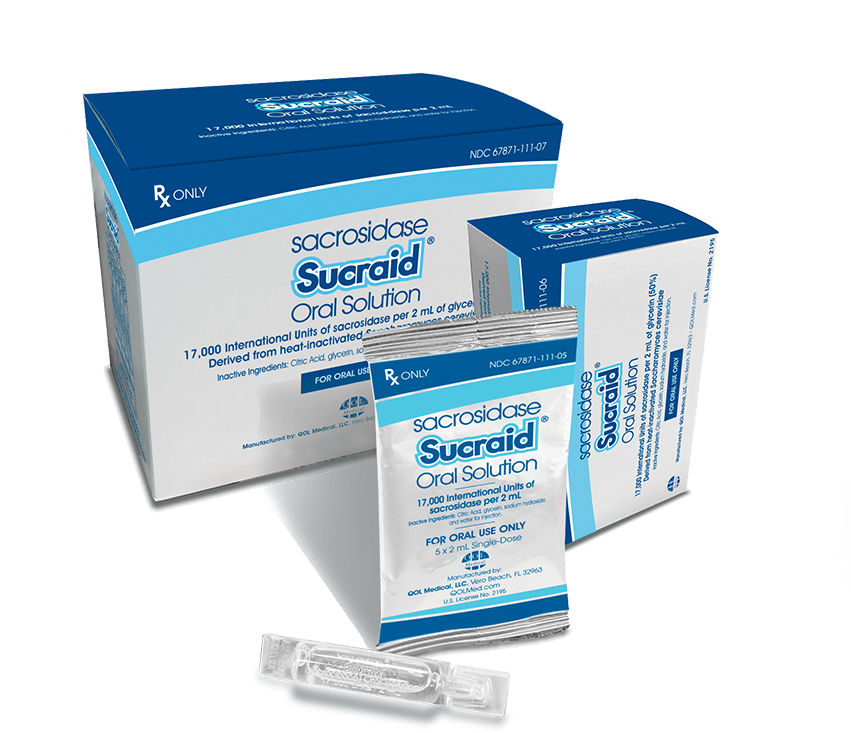
- Three-day room temperature stability at 59°F to 77°F (15°C to 25°C)
- No measuring required. Just twist, squeeze, and mix.
- Small enough to fit in your pocket.
Recommended dosing for Sucraid® single-use containers:
The contents of the Sucraid® 2-ml single-use container should be diluted in 4 ounces of water, milk, or infant formula. Patients should consume:
- All of the mixed solution dose per meal or snack for patients over 33 lb (15 kg) in body weight
- Half of the mixed solution dose per meal or snack for patients 33 lb (15 kg) and less
- For patients 33 lb (15 kg) and less using the Sucraid® 2-ml single-use container, the second half of the mixed solution should be refrigerated and discarded if not used within 24 hours
See prescribing information for complete dosing instructions.
Meet the Sucraid® Patients
Hear more about Sucraid® from the patients who take it.
[Now] I can eat more and not experience symptoms, so I don’t dislike eating. [CSID] is a disease that can be tested for, and can be managed.
Stephanie
Play Video
Addie started Sucraid® when she was 21 months old. For us, it was an opportunity to see hope. There’s a light at the end of this tunnel. She’s going to be okay.
Addie’s Parents
Individual results may vary.
Play Video
It came back that James had CSID… After we got a prescription for Sucraid®… we saw symptoms improving. Over time he was symptom-free.
James’s Parents
Individual results may vary.
Play Video
These statements reflect individual experiences and should not be used as a substitute for talking with your doctor about whether Sucraid® is right for you. Your doctor can discuss the benefits and risks with you. Each patient experience is unique and QOL Medical, LLC does not provide any warranty or guarantee as to the response a patient will have to Sucraid®.
References
- Sucraid® [package insert]. Vero Beach, FL: QOL Medical, LLC; 2024.
- Treem WR, McAdams L, Stanford L, Kastoff G, Justinich C, Hyams J. Sacrosidase therapy for congenital sucrase-isomaltase deficiency. J Pediatr Gastroenterol Nutr. 1999;28(2):137-42. doi:10.1097/00005176-199902000-00008